QEMSCAN mineral identification is performed online during sample measurement. If X-ray raw data are saved, mineral identification can be re-run offline using different mineral identification rule sets. QEMSCAN mineral identification is performed in two steps:
1) Elemental identification and quantification by the Spectral Analysis Engine (SAE)
2) Matching of elemental concentration ranges with phase (mineral) definitions in the Species Identification Protocol (SIP)
QEMSCAN mineral quantification is performed offline in iDiscover.
Spectral Analysis Engine
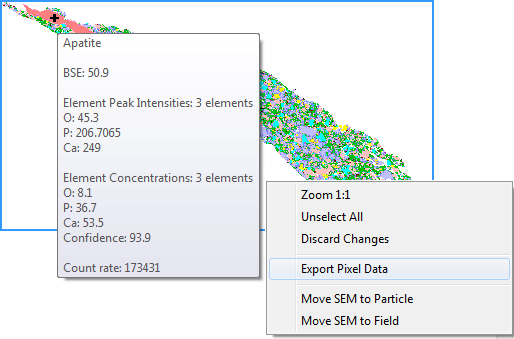
The SAE is fitting up to 72 pure elemental spectra, measured on a given SEM platform-EDS detector configuration, into a measured low-count energy-dispersive X-ray (EDX) spectrum. The SAE so called ‘element concentration’ approach then calculates the best match, by a) recording the presence of elemental spectra, and b) quantifying the relative contribution of each elemental spectra in the measured spectrum. The quality of the spectral match, as well as the measured X-ray count rate and backscatter brightness (BSE), is also recorded. The ‘elemental concentration’, or more specifically the relative contribution of elemental spectra in a measured mineral EDX spectrum, is different to the elemental mass percentage of the mineral. In the dolomite example below, the elemental weight percentages are Ca 21.7, Mg 13.2, C 13.0, and O 52.1, while ‘elemental concentrations’ are given as Ca 12.8, Mg 21.2, C 12.8, and O 23.1.
If all elements are selected for the spectra fitting operation, computing time increases. More importantly, noise is being introduced by an increasing number of partially overlapping elemental spectra. Overlapping element and element substitution rule sets are in place to limit element mismatches. If elements present in a measured spectrum have been disabled, the result would be a poor spectral match. Best results are achieved if the list of enabled elements coincides with those present in the measured sample. For O&G applications, the following 19 elements are commonly selected: C, O, Na, Mg, Al, Si, P, S, Cl, K, Ca, Ti, Cr, Mn, Fe, Cu, Zr, Ba.
Species Identification Protocol
The elemental ‘concentrations’ reported by the SAE for each individual measurement point (EDX spectrum) are compared online to the Species Identification Protocol, a list of phase definitions commonly referred to as ‘SIP list’. A measured spectrum is being assigned to a single phase, if it matches all criteria of the phase definition. Mineral phase definitions include ‘must have’ and optional ‘may have’ elemental ranges. Elemental ranges reflect the fact that multiple iterations of low-count spectra (typically 1,000 X-rays) of a single high-count spectrum necessarily results in statistical variation. In the example below, simulated low-count ranges of the high-count spectrum fit are provided for the four elements that make up dolomite: Ca 32-53, Mg 14-28, C 7-20, and O 15-32. Elemental ranges can also be used to account for natural chemical variation in a mineral. However, significant chemical variations are best approached by defining multiple end member SIP entries for a given phase. In addition to elemental ranges, elemental ratios and more complex formula can be set as rules in SIP definitions. Furthermore, optional thresholds for BSE brightness, X-ray count rate, and spectral match quality (aka ‘composition confidence’) can be defined.
In contrast to the best-match elemental fitting approach in the SAE, phase identification in the SIP is performed on a first-match basis. Phase definitions are therefore position dependent. The measured elemental concentration, BSE brightness, count rate and spectral match data of a measured spectrum is sequentially compared to all phase definitions, and mapped to the first in the SIP list that provides a match. If a measured data point does not match any predefined entry, it remains unclassified and will be reported as ‘Others’.
Expertise in SIP development is exercised by establishing elemental ranges that reliably capture all the variability inherent in low-count spectra, while preventing phase definitions to become too broad and potentially capturing spectra of non-identical phases. This expertise is often treated as valuable IP by some QEMSCAN service providers. A number of software tools are available in iDiscover, the QEMSCAN expert analysis and reporting software component, to facilitate this task. A layered approach to SIP development has previously been presented (Haberlah et al., 2011), utilising the sequential SIP approach to its full advantage. However, mineral phases characterised by large chemical variability such as some clay minerals, or mixed spectra obtained from excitation volumes that include multiple minerals, traditionally addressed as a ‘boundary phases, can remain a challenge. Boundary phases between up to four minerals can be assigned to neighbouring minerals in a statistically correct and controlled way by applying rules defined in the ‘Boundary Phase Processor’.
Primary Mineral List
Once all phases have been defined on a pixel-by-pixel basis by online SIP classification and offline application of pre-processors such as the ‘Boundary Phase Processor’, individual phases need to be grouped into real minerals or phases of interest in order to be reported as volume or weight percentage contributions. Conversion of measured phase data into chemical assays reports can be of further interest. Both are performed by grouping similar SIP phases in the ‘Primary Mineral List’ and assigning them a single density and chemical composition. In our example, dolomite would be assigned a bulk density of 2.83 g/cc and a chemical composition of weight percentages Ca 21.73, Mg 13.18, C 13.03, and O 52.06. For analysis and reporting purposes, multiple Primary Mineral List entries can further be grouped into Secondary Mineral List entries. For example, an Fe-rich version of dolomite will require a separate SIP entry for identification, and Primary Mineral List entry for adequate chemical and density characterisation. However, both dolomite entries can be grouped in the final modal mineralogy and elemental assay reports without compromising reported accuracy in composition.
The task of assigning relevant compositional data to identified phases requires a good understanding of the chemical variability inherent in some minerals comprising the sample. This task can be facilitated by including bulk compositional data from X-Ray Fluorescence (XRF) analysis. QEMSCAN software tools, such as the ‘Assay Reconciliation Report’, assist with the task of optimising chemical composition and density assumptions. Some applications in O&G are best advised to limit modal mineralogy reports to volume percentages as opposed to weight percentages, if the density values for identified phases are poorly constrained.
Sample presentation
Any discussion of automated mineralogy mineral identification and quantification would be incomplete without highlighting the impact of sample preparation and measurement setup on the results and data interpretation. Sample preparation and selected measurement area and EDX acquisition setup ultimately define reported results and how representative these are of the sample at large.
References
Haberlah, D., Owen, M., Botha, P.W.S.K., Gottlieb, P., 2011. SEM-EDS based protocol for subsurface drilling mineral identification and petrological classification, in: Broekmans, M.A.T.M. (Ed.), Proceedings of the 10th International Congress for Applied Mineralogy (ICAM), 01 05 August 2011, Trondheim, Norway. Trondheim, Norway, pp. 265–273.
 |
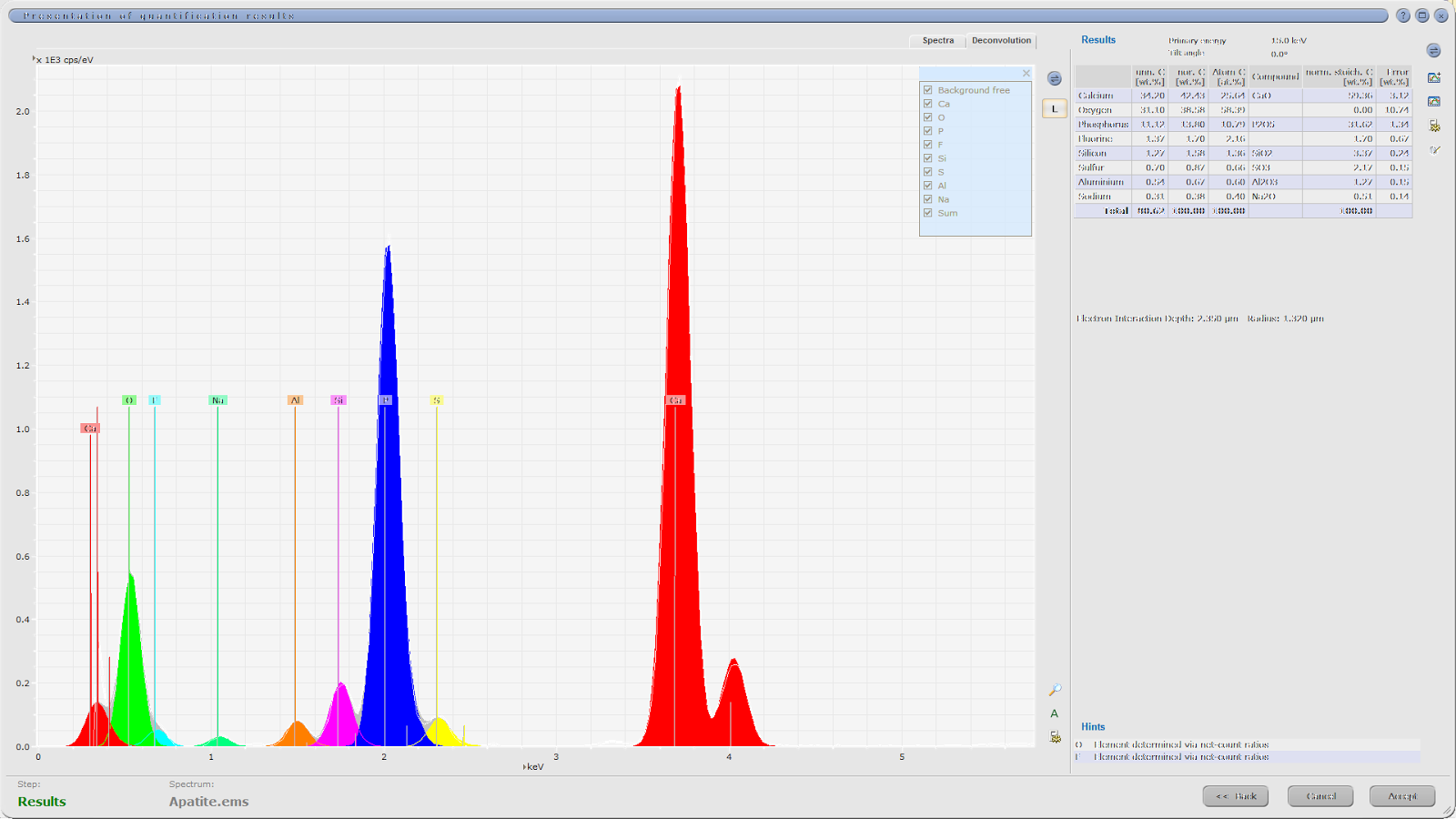
Example illustrating the fitting of elemental spectra (coloured) into a
(high-count) measured dolomite spectrum (grey). The nominal elemental weight percentage
of dolomite is provided, as well as the ‘concentrations’ or relative
contributions of elemental spectra providing the best fit. Finally, simulated
ranges for 1,000 X-rays low-count spectra are given, which would be used in the
SIP entry to reliably identify the measured spectrum as dolomite.
|